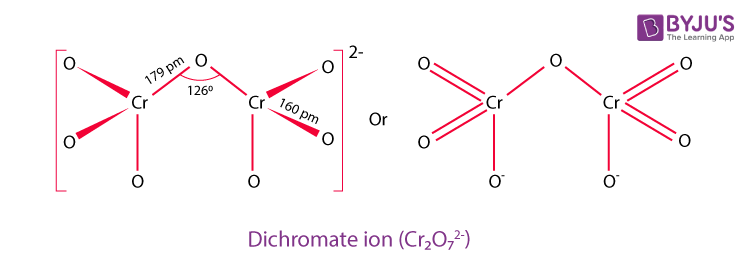
Aqueous-Phase Nanomolar Detection of Dichromate by a Recyclable Cd(II) Metal–Organic Framework | Crystal Growth & Design

Balance the following redox reactions by ion electron method. Cr2O^2 - 7 + SO2(g)→ Cr^3 + (aq) + SO^2 - 4(aq) (in acidic solution)

Balance the following redox reaction by using Oxidation number method in acidic medium.Cr2O7^-2(aq)+SO3^2(aq)rarrCr^+3(aq)+SO4^-2(aq)

Another Look at Photoelectron Spectra of the Anion Cr2O2–: Multireference Character and Energetic Degeneracy | Journal of Chemical Theory and Computation

Balance the following reaction by oxidation number method: K2Cr2O7 + FeSO4 + H2SO4→ K2SO4 + Cr2(SO4)3 + Fe2(SO4)3 + H2O .

Another Look at Photoelectron Spectra of the Anion Cr2O2–: Multireference Character and Energetic Degeneracy | Journal of Chemical Theory and Computation

Visible-Light-Promoted Photocatalytic Applications of Carbon Dots: A Review | ACS Applied Nano Materials

Another Look at Photoelectron Spectra of the Anion Cr2O2–: Multireference Character and Energetic Degeneracy | Journal of Chemical Theory and Computation

Another Look at Photoelectron Spectra of the Anion Cr2O2–: Multireference Character and Energetic Degeneracy | Journal of Chemical Theory and Computation